Robust Development Pipeline
As a result of the Company’s enabling technologies, InMed has identified three drug candidates that are currently at various stages of development:
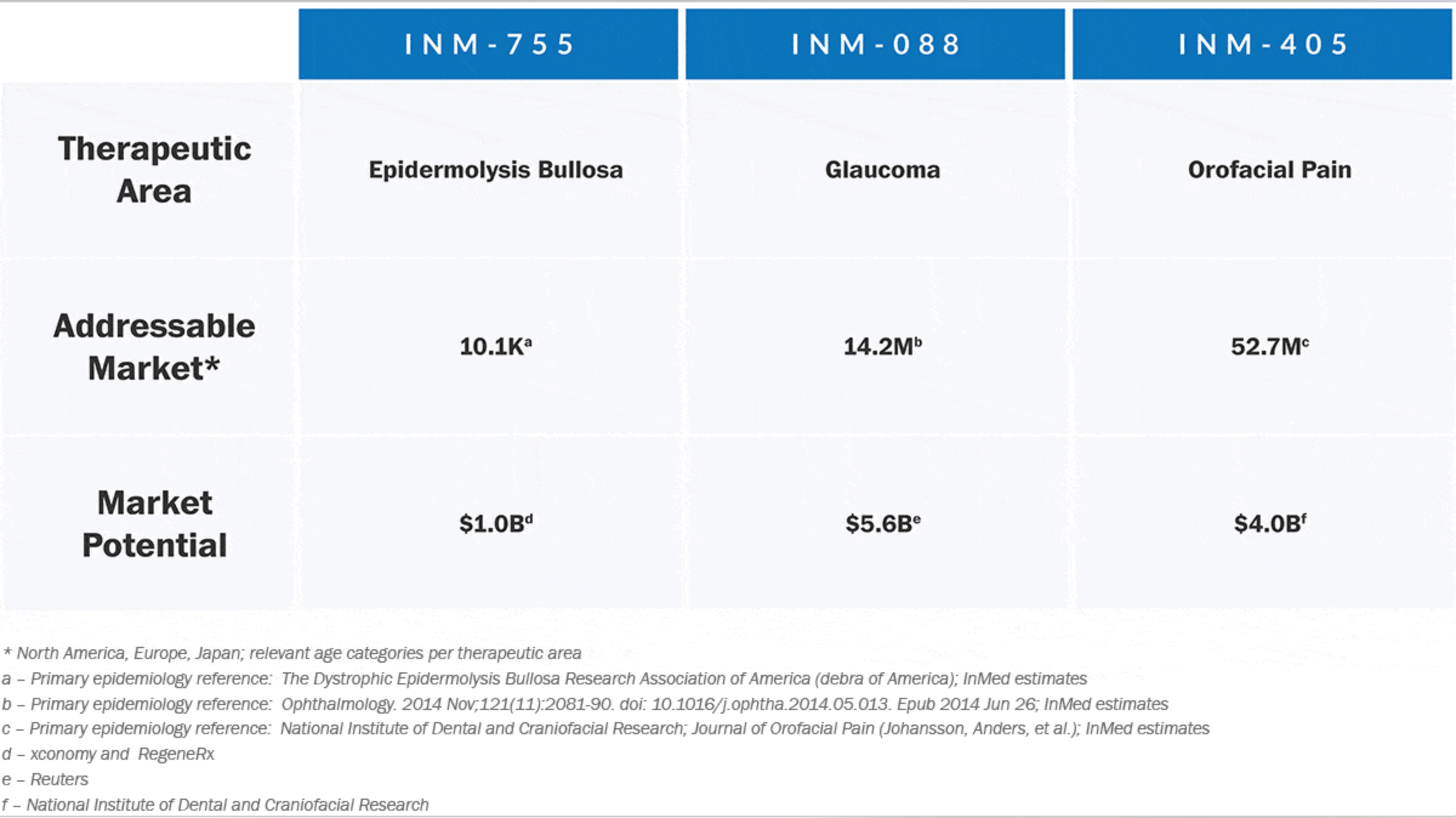
- INM-755, our lead product in Phase 1 development in healthy volunteers. INM-755 is under development for the treatment of epidermolysis bullosa (“EB”), a severe genetic skin disorder (according to analyst reports, which range widely, there are between 10,000 and 50,000 estimated EB patients in North America, Europe and Japan and potential global market revenues of up to $1.0 billion for EB related drugs/treatments);
- INM-088, in advanced preclinical development for the treatment of glaucoma, the second leading cause of blindness in the developed world (according to a February 2015 article published by Reuters, there is a global market of more than $5.6 billion for glaucoma related drugs/treatments); and
- INM-405, in development as a topical treatment for localized pain, specifically orofacial pain (the global market of which is estimated to exceed $4.0 billion, according to the NIH).