INM-901 for Alzheimer's disease
InMed’s INM-901 is a novel, small molecule drug candidate in development for the treatment of Alzheimer’s Disease.
INM-901 demonstrates novel therapeutic effects from current treatments
InMed’s INM-901 is a drug candidate being developed as a potential multi-factorial treatment for Alzheimer’s disease. Promising studies demonstrate INM-901’s neuroprotective effects and an ability to extend neurite length, signifying improved neuronal function, a unique therapeutic effect not shown in any current Alzheimer’s disease treatments.
In a recent preclinical study, INM-901 demonstrated a reduction in several plasma and brain markers of neuroinflammation, a recognized contributor to Alzheimer’s disease development and progression.
INM-901 demonstrates promising disease-modifying effects in Alzheimer's disease studies
InMed has conducted several in vitro and in vivo studies to test the pharmacological effects of INM-901, a cannabinoid analog, in Alzheimer’s disease preclinical models with promising results demonstrating disease-modifying effects.
A summary of INM-901 preclinical study results:
- INM-901 displayed neuroprotective effects by reducing cell death in an amyloid-beta-induced cytotoxicity study
- INM-901 reduces neuroinflammation, including significantly reducing pro-inflammatory cytokines associated with Alzheimer’s
- demonstrated an ability to promote neurite outgrowth, signifying the potential to improve neuronal function, a potential breakthrough in the treatment of Alzheimer’s disease
- INM-901 demonstrates a trend in improvement in cognitive function and memory, locomotor activity, anxiety-based behavior, sound awareness and neuronal function
- Additional mRNA data supports the observations made in behavior studies in locomotor activity, cognition and memory.
Most recently, InMed conducted longer-term in vivo studies with INM-901 demonstrating favorable behavioral outcomes, confirming previous short-term pilot study data.


INM-901 targets several biological pathways associated with Alzheimer’s disease
INM-901 potentially offers a unique treatment approach that may target several biological pathways associated with Alzheimer’s disease.
In a well-characterized in vivo Alzheimer’s disease study models, it was observed that INM-901 is a preferential signaling agonist of cannabinoid receptor 1 (CB1), cannabinoid receptor 2 (CB2) and impacts the peroxisome proliferator-activated receptor (PPAR) signaling pathway.
CB1 and CB2 receptors are both part of the endocannabinoid system and are found throughout the body, including in the brain. CB1 receptors are primarily located in the central nervous system, particularly in areas involved in memory, cognition and motor function, while CB2 receptors are involved in modulating neuroinflammation and immune responses.
Activation of CB1 and CB2 receptors has been shown to have neuroprotective effects, meaning they can help protect brain cells from damage and death. In Alzheimer’s disease, where neuronal cell death is a hallmark feature, enhancing the activity of these receptors may help to slow down the progression of the disease. Activation of these receptors has also been shown to have an impact on neuroinflammation. As neuroinflammation is also believed to contribute to the progression of Alzheimer’s disease, targeting these receptors could help alleviate this inflammatory response. While further research is needed to fully understand the role of CB1 and CB2 receptors in Alzheimer’s disease, targeting these receptors could offer novel therapeutic strategies for the treatment of this devastating condition. (1)(2)
Peroxisome proliferator-activated receptors (PPARs) are also considered potential therapeutic targets for neurodegenerative disorders such as Alzheimer’s disease. PPAR’s are ligand-activated transcription factors of nuclear hormone receptor superfamily comprising of the following three subtypes: alpha (“PPAR-α”), gamma (“PPAR-γ”), and beta/delta (“PPAR-β/δ”). Activation of PPAR-α reduces triglyceride level and is involved in regulation of energy homeostasis. Activation of PPAR-γ causes insulin sensitization and enhances glucose metabolism, whereas activation of PPAR-β/δ enhances fatty acids metabolism. (3)(4)
(1) Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease
(2) Cannabinoid CB2 Receptors in Neurodegenerative Proteinopathies: New Insights and Therapeutic Potential
(3) The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases
(4) PPARs in the central nervous system: roles in neurodegeneration and neuroinflammation
INM-901 can be utilized as an oral formulation
InMed conducted preclinical studies confirming that INM-901 can be administered orally and achieve therapeutic levels in the brain comparable to those obtained through intraperitoneal (“IP”) injection, which is a common route of administration for preclinical investigation of neurodegenerative diseases. The data indicates the INM-901 formulation can be administered orally and maintains a similar drug exposure levels as IP delivery over a 24-hour period in the brain. This oral delivery method offers potential advantages such as reduction in treatment delivery costs.
One of the main challenges of the newly-approved disease-modifying monoclonal anti-bodies is their drug delivery limitations. As large molecules, the monoclonal antibodies are administered via intravenous infusions every two to four weeks typically at a medical site by trained staff.
The drug delivery options of small molecules could greatly reduce treatment costs and the compliance challenges associated with large molecule antibody therapies.

INM-901 next steps - advancing preclinical studies
InMed is accelerating development of its Alzheimer’s disease program.
The Company is conducting further molecular analyses to better define the mechanisms of action and potential role of INM-901 in Alzheimer’s treatment. The analyses will focus on the following areas using mRNA, protein and histological measurements:
- Receptor engagement: CB1/CB2 and PPAR;
- Neuroinflammation: various cytokines and inflammatory marker protein level;
- Neurogenesis: assess markers for neuronal differentiation and neuronal function; and
- Neuroprotection: evaluating stress responses and cellular growth/survival.
Additionally, the development of the chemistry, manufacturing, and controls (CMC) for both the drug substance and the drug product formulation is ongoing, with GLP studies in the planning stages to support an IND submission.

Alzheimer's disease program progress
In addition to the promising in vivo studies in Alzheimer’s disease, InMed is building a strong foundation to support the success of its INM-901 program has progressed follow on several developments in the program:
- International patent – Several patents have been filed by InMed including an international application citing the use of rare cannabinoids and analogs for the potential treatment of neurodegenerative diseases such as Alzheimer’s Disease, Parkinson’s Disease, Huntington’s Disease and others. One patent specifies such compounds that may inhibit or slow the progression of neurodegenerative diseases by providing neuroprotection in a population of affected neurons.
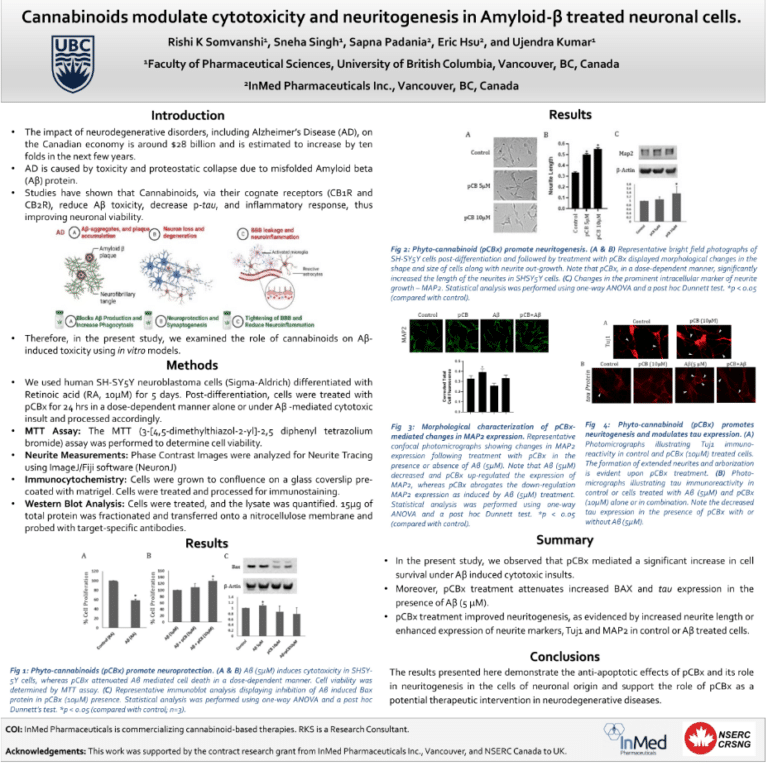
- Presentation at Canadian Neuroscience Meeting – An InMed sponsored scientific poster, entitled “Cannabinoids modulate cytotoxicity and neuritogenesis in Amyloid-beta-treated neuronal cells,” demonstrated the ability of a specific rare cannabinoid to reduce amyloid toxicity and tau protein expression while enhancing neuronal cell growth and neuritogenesis markers in vitro, all considered to be important targets in the potential treatment of neurodegenerative diseases such as Alzheimer’s.
- Addition of leading Alzheimer’s disease expert, Dr. David G. Morgan, to InMed Scientific Advisory Board. Dr. Morgan is Director of the Alzheimer’s Alliance and MSU Foundation Professor of Translational Neuroscience at Michigan State University. He is internationally recognized for his work on immunotherapy and gene therapy to treat the Alzheimer-related pathologies.
Candidate selection process
InMed’s research into the neuroprotective effects of INM-088 for glaucoma led to the screening of multiple cannabinoids against a panel of non-ocular neuron models, including brain neurons. One cannabinoid, in particular, emerged as a promising candidate for protection against neurodegenerative diseases.
InMed’s team developed several analogs of this cannabinoid to potentially improve the effects demonstrated in the original neuronal in vitro models. Further screening in several Alzheimer’s disease assays identified two analogs that were advanced into in vivo preclinical testing involving a well-established Alzheimer’s proof-of-concept model to measure drug impact on several disease characteristics. Based on the results from this battery of preclinical testing, INM-901 has been selected as InMed’s lead drug candidate for continued pharmaceutical R&D studies in Alzheimer’s disease.

Alzheimer’s disease – a major unmet medical need
Newly-approved Alzheimer’s disease medications primarily address symptoms related to memory and cognitive function via the reduction of beta-amyloid plaques. Some may slow the rate of cognitive decline, but no treatment has shown to reverse its effects. These medications are aimed at removing amyloid plaque build-up between the neurons in the brain; however, they do not restore or rebuild deteriorating neurons and thus do not reverse Alzheimer’s disease progression. In addition, these treatments are related to some significant side effects, including inflammation and bleeding in the brain, requiring brain scans once or twice a year. The administration of these treatments, which include an intravenous infusion every 2-4 weeks, also presents a challenge.
Cannabinoids and their potential role in neuronal disorders
Several in vitro and in vivo studies published by third parties support InMed’s findings of the effects of rare cannabinoids in neuronal disorders. Rare cannabinoids may be promising drug candidates for the treatment of neurodegenerative diseases due to their pharmacological effects.
- Ability to cross the blood-brain barrier: The blood-brain barrier is the specialized system of brain microvascular endothelial cells that serves to regulate several functions including shielding the brain from toxic substances (including viruses, bacteria and other foreign substances including many drugs), supplying brain tissues with nutrients and filtering harmful compounds from the brain back into the bloodstream. Rare cannabinoids, including INM-901, are highly lipophilic (dissolves readily in fats, oils and lipids) and can easily cross the blood-brain barrier, making them potential pharmaceutical agents for neurological disorders.
- Targeting several receptor systems: In addition to the endocannabinoid system, rare cannabinoids are capable of targeting multiple receptor systems which may be beneficial as a multi-pronged approach to treat complex diseases of the brain.

Rare cannabinoid pharmaceutical pipeline
INM-901, a drug candidate for Alzheimer’s disease, is one of three pharmaceutical programs being developed by InMed. INM-755 cannabinol (CBN) cream has completed a Phase 2 clinical trial and the Company will evaluate strategic partnership for further development in the treatment of EB or other itch-related skin conditions. INM-089 is a cannabinol analog being developed for the treatment of age-related macular degeneration.




