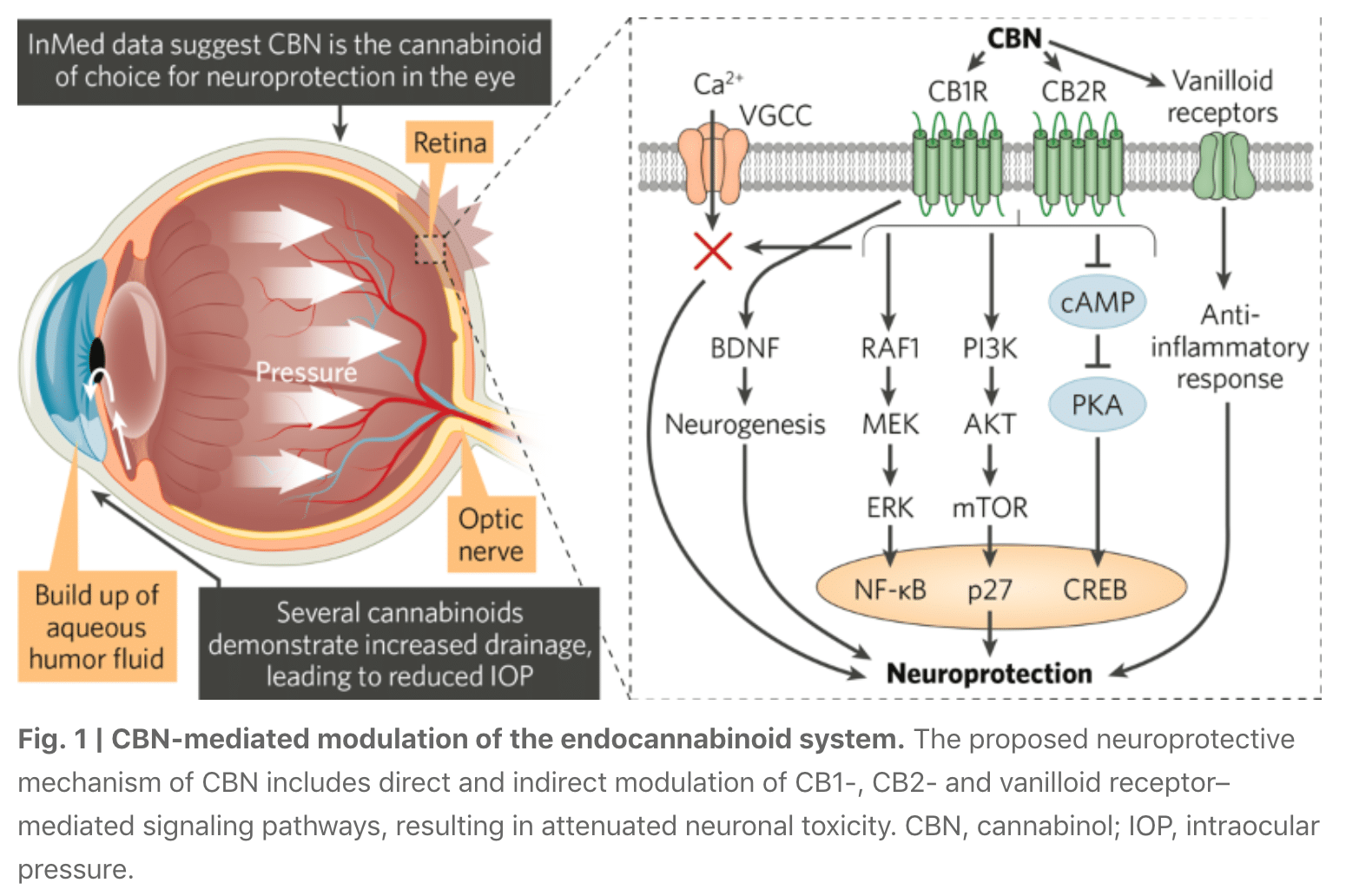
InMed Pharmaceuticals, a clinical stage pharmaceutical company based in Vancouver, Canada, is developing rare cannabinoid–based therapeutic drug candidates for patients who may benefit from the unique physiological effects of these compounds. Specifically, InMed is exploring the therapeutic potential in ocular disease of cannabinol (CBN), a non-psychoactive rare cannabinoid with neuroprotective, anti-inflammatory, anti-nociceptive and intraocular pressure (IOP)-reducing effects. InMed has a lead drug candidate in clinical development—INM-755, a CBN-based topical dermatological cream for the treatment of epidermolysis bullosa; a second drug candidate in preclinical development—INM-088, a CBN-based topical eyedrop formulation for the treatment of glaucoma; and additional discovery programs utilizing CBN and other rare cannabinoids. This growing pipeline places InMed at the forefront of pharmaceutical companies harnessing rare cannabinoids for treating a range of disorders that are partially or wholly regulated by the endocannabinoid receptor system, as well as other systems that may be modulated by cannabinoids.
“While THC and CBD have grabbed most of the attention around the medical use of cannabinoids, our focus is on research of rare cannabinoids in various diseases as potential therapeutic candidates,”said Eric A. Adams, InMed’s CEO and President. “At InMed, we are initially focusing on the unique physiological effects of cannabinol, a compound we have shown to have distinct advantages over other cannabinoids, as well as other classes of molecules as a potential treatment for conditions such as epidermolysis bullosa and glaucoma.”
Read the full feature article here in Nature BioPharma DealMakers.