Vancouver, BC – May 14, 2020 – InMed Pharmaceuticals Inc. (“InMed” or the “Company”) (TSX:IN; OTCQX:IMLFF), a clinical-stage pharmaceutical company developing medications targeting diseases with high unmet medical need and leading the way in the clinical development of cannabinol (“CBN”), today reported financial results for the third quarter of fiscal year 2020 (“3Q20”) which ended March 31, 2020.
Conference Call & Webcast:
| Thursday, May 14, 2020 at 8:30 AM Pacific Time, 11:30 AM Eastern Time
Local – Toronto (+1) 416-764-8609 Local – Vancouver (+1) 778-383-7417 Toll Free – North America (+1) 888-390-0605 Conference ID: 43237343 Webcast: https://produceredition.webcasts.com/starthere.jsp?ei=1314789&tp_key=2a52cb9b61 |
Replays, Available through May 21, 2020:
|
Toronto: |
(+1) 416-764-8677 |
|
|
North America (Toll Free): |
(+1) 888-390-0541 |
|
|
Playback Passcode: |
237343 # |
|
“In the third quarter of fiscal 2020, InMed made important strides in both its therapeutic development and cannabinoid biosynthesis programs,” stated InMed President and Chief Executive Officer, Eric A. Adams. “We continue to lead the clinical development of cannabinol as a potential therapeutic option for skin and ocular diseases as demonstrated by completion of our first Phase 1 trial with INM-755 and ongoing preclinical and formulation work with INM-088. We are now well underway toward the initiation of our second Phase 1 trial for INM-755 with acceptance of the Clinical Trial Application (“CTA”) last month. In addition, as announced earlier this week, we are continuing to strengthen our patent position for therapeutic cannabinoid applications including the potential treatment of glaucoma. Meanwhile, we are pleased with the progress our scientific team has been making, working behind the scenes with world-class organizations such as the Almac Group (UK) (“Almac”), towards innovative cannabinoid manufacturing methods.”
Research & Development Update:
- INM-755 for the treatment of epidermolysis bullosa (“EB”). On April 1, 2020, InMed announced completion of treatment and clinical evaluation in the 755-101-HV Phase 1 trial with INM-755, a CBN skin cream. One week prior to that date, with no interim adverse events having been observed in the 755-101-HV trial in healthy volunteers on intact skin that would preclude further development, InMed filed a CTA for its second Phase 1 trial in healthy volunteers (755-102-HV). This second trial will examine the safety of INM-755 on epidermal wounds. On April 30, 2020, InMed announced approval of this second CTA. InMed is actively working with its clinical trial partners at the Centre for Human Drug Research in the Netherlands to prepare for subject enrollment in Study 755-102-HV as expediently as reasonable. Final results from the 755-101-HV trial are anticipated to be announced in the third quarter of calendar 2020.
- INM-088 for the treatment of glaucoma. InMed has completed in vitro and in vivo testing and is near completion of formulation study data analysis for INM-088. InMed has continued to firm up its intellectual property position via the filing of a Patent Cooperation Treaty, or PCT, application for the neuroprotective effect of CBN. Next steps in the INM-088 program include selection of a final delivery technology and conducting additional in vivo studies, if needed. Depending on the results of those studies, InMed continues to anticipate commencement of IND-enabling preclinical toxicology studies in the second half of calendar 2020.
- Biosynthesis manufacturing technology. InMed recently announced that it is working with Almac in developing innovative methods for low-cost, high yield and pharmaceutical-grade cannabinoid manufacturing. Specifically, InMed is combining its scientific expertise with Almac’s recognized leadership in enzyme engineering, process-development and good manufacturing practice (“GMP”) manufacturing capability to enable InMed’s biosynthesis program. Based on the current plan, InMed anticipates this process to be GMP-batch ready in 4Q of calendar 2020.
Results of Operations (expressed in Canadian Dollars):
- For the three and nine months ended March 31, 2020, the Company recorded a net loss of $2.81 million and $9.54 million, or $0.02 and $0.06 per share, compared with a net loss of $3.49 million and $8.99 million, or $0.02 and $0.05 per share, for the three and nine months ended March 31, 2019.
- Research and development expenses were $1.59 million for 3Q20, compared with $1.62 million for the three months ended March 31, 2019. For the nine months ended March 31, 2020, research and development expenses totaled $5.85 million, which compares with $3.19 million for the comparable period in fiscal 2019. While research and development expenses for the three months ended March 31, 2020 and the three months ended March 31, 2019 were fairly equivalent, the increase in research and development expenditures between the nine months ended March 31, 2020 as compared to the equivalent period in fiscal 2019, did see a large increase. This increase of $2.66 million between these nine-month periods was primarily due to increased spending on INM-755 preclinical safety pharmacology and toxicology studies, manufacturing costs for INM-755 material to be used in the Phase 1 clinical trials and the commencement of the first Phase 1 trial. In addition, the Company incurred increased salaries and benefits commensurate with the increase in research and development activities.
- The Company incurred general and administrative expenses of $1.04 million for 3Q20, compared with $0.99 million for the three months ended March 31, 2019. For the nine months ended March 31, 2020, general and administrative expenses totaled $2.93 million, which compares with $2.72 million for the comparable period in fiscal 2019. The increase in general and administrative expenses for both the three and nine months to March 31, 2020 was primarily due to increased accounting and legal expenses pertaining to certain corporate initiatives and increased salaries and benefits offset by decreased investor relation related expenditures.
- The Company also incurred non-cash, share-based payments, in connection with the grant of stock options, of $0.37 million for 3Q20, compared with $0.89 million for the three months ended March 31, 2019. For the nine months ended March 31, 2020, non-cash, share-based payments totaled $0.96 million, which compares with $3.34 million for the comparable period in fiscal 2019.
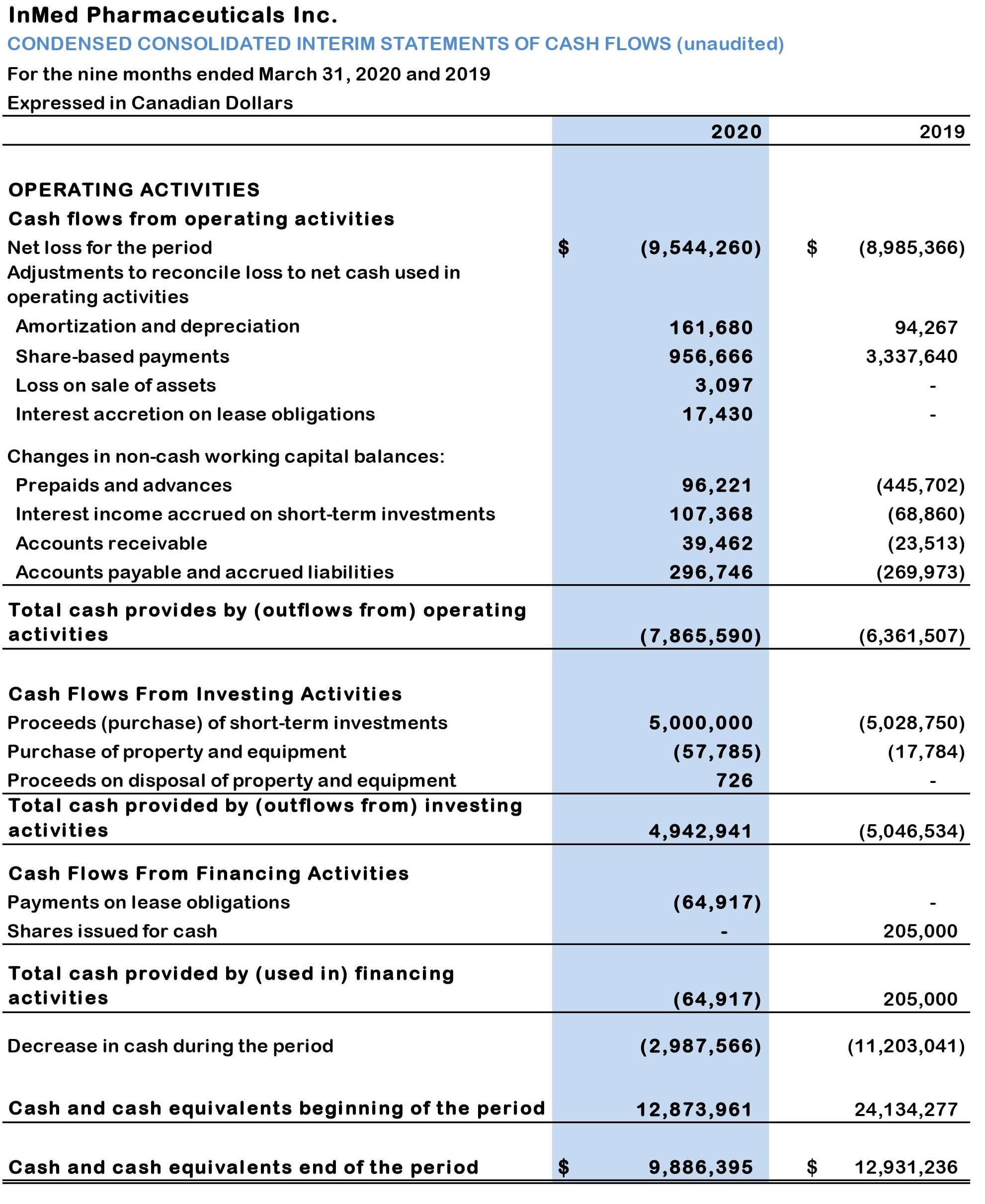
- At March 31, 2020, the Company’s cash, cash equivalents and short-term investments were $9.94 million, which compares to $18.04 million at June 30, 2109 and $12.01 million at December 30, 2019. The decrease in cash, cash equivalents and short-term investments during the nine months to December 31, 2019, was primarily due to cash outflows from operating activities.
- At March 31, 2020, the Company’s total issued and outstanding shares were 172,283,633. In addition, at March 31, 2020, there were 17,717,641 warrants, expiring June 2020, with a weighted average price of $1.24 and 19,462,500 outstanding stock options with a weighted average exercise price of $0.45.
Table 1: Condensed consolidated statements of financial position (unaudited):
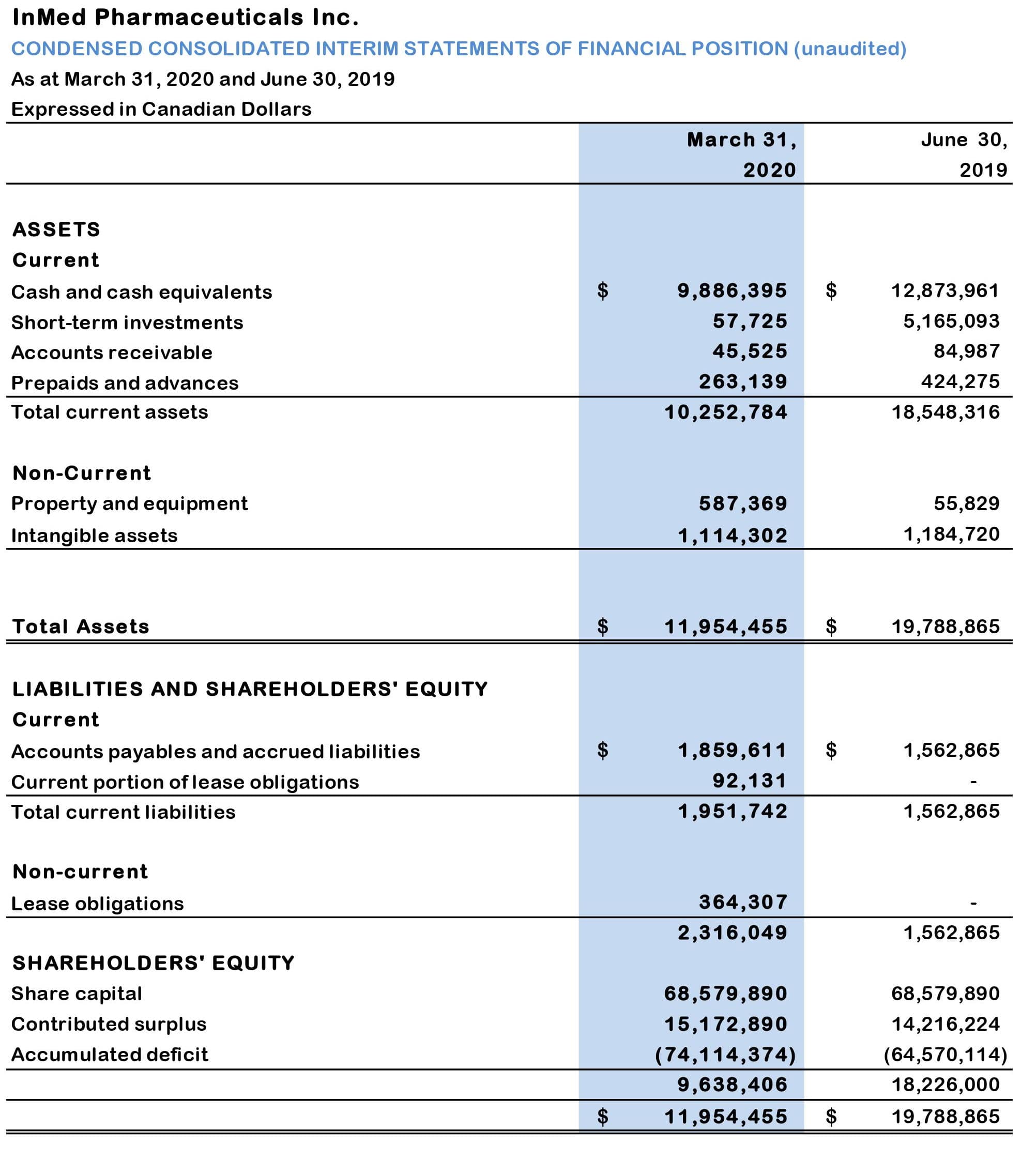
Table 2: Condensed consolidated statements of operations and comprehensive loss (unaudited):
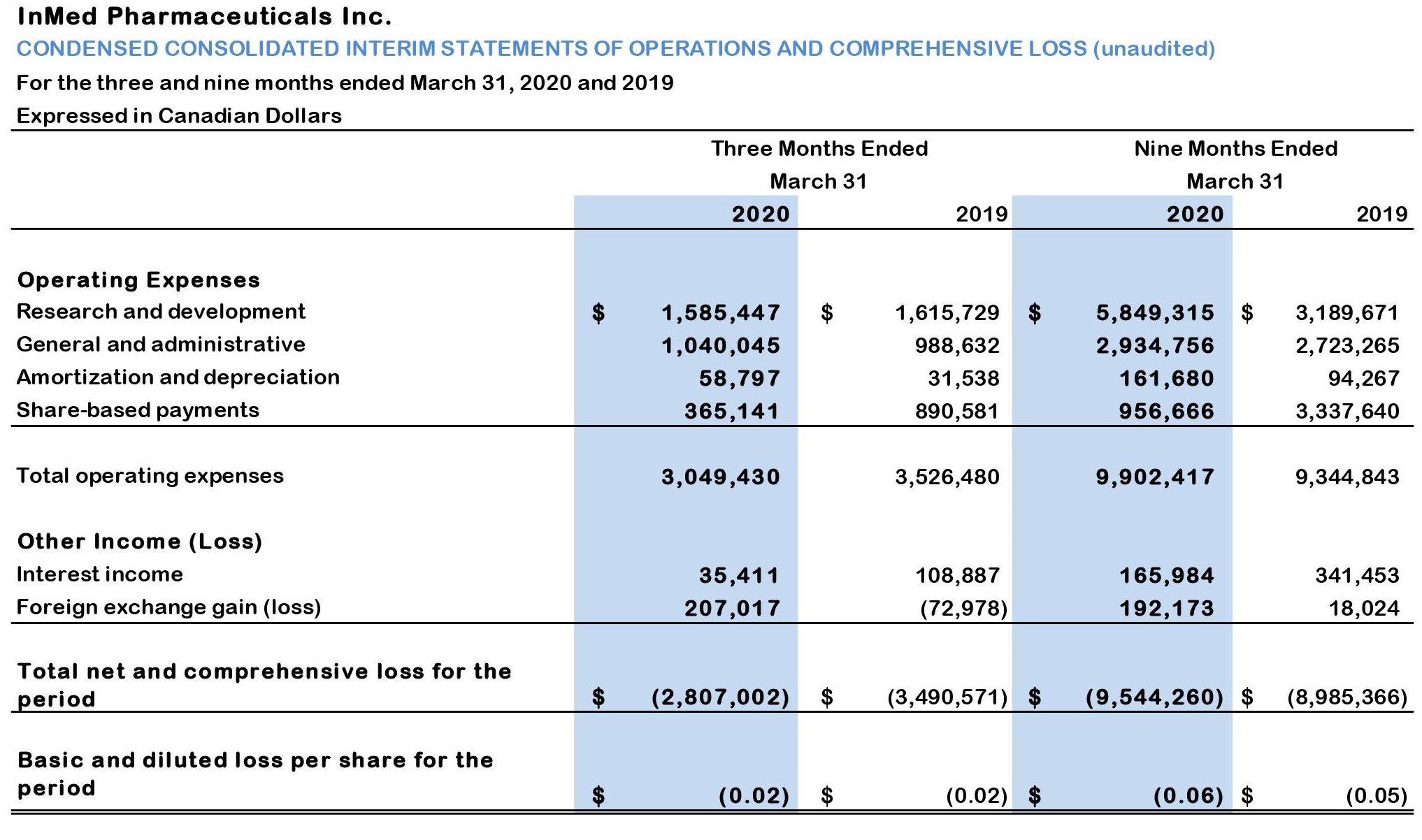
Table 3: Condensed consolidated interim statements of cash flows (unaudited):
About InMed: InMed Pharmaceuticals is a clinical stage biopharmaceutical company developing a pipeline of cannabinoid-based medications, initially focused on the therapeutic benefits of cannabinol (CBN) in diseases with high unmet medical needs. The Company is dedicated to delivering new therapeutic alternatives to patients that may benefit from cannabinoid-based medicines. For more information, visit www.inmedpharma.com.
About Cannabinol (CBN): CBN is a rare cannabinoid with unique physiological properties that may result in distinct therapeutic and safety characteristics relative to the more commonly known cannabinoids tetrahydrocannabinol (THC) and cannabidiol (CBD). InMed Pharmaceuticals is exploring the therapeutic potential of CBN in diseases with high unmet medical need.
About INM-755: INM-755 is a CBN cream intended as a topical therapy to treat epidermolysis bullosa (EB) and potentially other dermatological diseases. Preclinical data demonstrate that INM-755 may help relieve hallmark EB symptoms, such as inflammation and pain, as well potentially restore the integrity of the skin in a subset of EB Simplex patients.
About Epidermolysis Bullosa (EB): EB is the collective name of a group of genetic disorders of connective tissues affecting individuals from birth and is characterized by fragile skin that is easily damaged, leading to extensive blistering and wounding. The blisters may appear in response to minor injury, even from heat, rubbing, scratching or adhesive tape. The disease has no definitive cure and all currently used treatments are directed towards symptomatic relief.
About INM-088: InMed is developing INM-088 as a CBN eye drop formulation targeting reduction of the intraocular pressure associated with glaucoma as well as being designed to serve as a neuroprotectant to the retinal ganglion cells.
About Glaucoma: Glaucoma is a group of eye conditions characterized by abnormally high pressure in the eye, which can damage the membranes of the retina and the head of the optic nerve, leading to blindness. Glaucoma is the second leading cause of blindness worldwide and can occur at any age but is more common in older adults.
Cautionary Note Regarding Forward-Looking Information:
This news release contains “forward-looking information” and “forward-looking statements” (collectively, “forward-looking information”) within the meaning of applicable securities laws. Forward-looking information is based on management’s current expectations and beliefs and is subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Forward-looking information in this news release includes statements about: leading the way in the clinical development of cannabinol (“CBN”); developing a pipeline of cannabinoid-based medications in diseases with high unmet medical need; and delivering new therapeutic alternatives to patients that may benefit from cannabinoid-based medicines; leading the clinical development of cannabinol as a potential therapeutic option for skin and ocular diseases; initiating the second Phase 1 trial for INM-755; continuing to strengthen our patent position for therapeutic cannabinoid applications including the potential treatment of glaucoma; developing innovative new cannabinoid manufacturing methods; examining the safety of INM-755 on epidermal wounds in the 755-102-HV trial; announcing final results from the 755-101-HV trial in the third quarter of calendar 2020; selecting a final delivery technology and conducting additional in vivo studies for the INM-088 program; commencing IND-enabling preclinical toxicology studies in the second half of calendar 2020 for INM-088; developing innovative methods for low-cost, high yield and pharmaceutical-grade cannabinoid manufacturing; having the biosynthesis process GMP-batch ready in the fourth quarter of calendar 2020; CBN having potential therapeutic advantages in specific disease models over certain cannabinoids; INM-088 reducing intraocular pressure and acting as a neuroprotectant to the retinal ganglion cells and optic nerve; INM-755 potentially relieving EB symptoms, such as inflammation and pain as well as potentially enhancing skin integrity in a subset of EB Simplex patients; and developing a proprietary biosynthesis manufacturing technology for the production of pharmaceutical-grade cannabinoids as well as a pipeline of medications targeting diseases with high unmet medical needs;.
With respect to the forward-looking information contained in this news release, InMed has made numerous assumptions regarding, among other things: continued and timely positive preclinical and clinical efficacy data; the speed of regulatory approvals; the ability to contract with suitable partners; demand for InMed’s products; and continued economic and market stability. While InMed considers these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties and contingencies.
Additionally, there are known and unknown risk factors which could cause InMed’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking information contained herein. Known risk factors include, among others: the outbreak and impact of COVID-19 may worsen; preclinical and clinical testing may not produce the desired results on a timely basis, or at all; regulatory applications may not be approved on a timely basis, or at all; cannabis licensing/importing issues may delay our projected development timelines; suitable partners may not be located; economic or market conditions may worsen; our existing cash runway may not allow us to complete our forthcoming significant milestones; the development of a proprietary biosynthesis manufacturing technology for the production of pharmaceutical-grade cannabinoids as well as a pipeline of medications targeting diseases with high unmet medical needs may not be as successful as desired, if at all. A more complete discussion of the risks and uncertainties facing InMed is disclosed in InMed’s most recent Annual Information Form and other continuous disclosure filed with Canadian securities regulatory authorities on SEDAR at www.sedar.com.
All forward-looking information herein is qualified in its entirety by this cautionary statement, and InMed disclaims any obligation to revise or update any such forward-looking information or to publicly announce the result of any revisions to any of the forward-looking information contained herein to reflect future results, events or developments, except as required by law.
NEITHER THE TORONTO STOCK EXCHANGE NOR ITS REGULATIONS SERVICES PROVIDER HAVE REVIEWED OR ACCEPT RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
SOURCE InMed Pharmaceuticals Inc.



